C: Case finding and confirm diagnosis
C: Case finding and confirm diagnosis | Evidence level | Strength of recommendation* |
|---|---|---|
| Smoking is the most important risk factor in COPD development. | I | Strong |
| Smoking cessation reduces mortality. | I | Strong |
| A thorough history and examination is the first step in COPD diagnosis. | III-2 | Strong |
| COPD is confirmed by the presence of persistent airflow limitation (post-bronchodilator FEV1/FVC <0.7). | III-2 | Strong |
| Diagnosis of COPD should be accompanied by regular assessment of severity. | III-2 | Strong |
| If FEV1 increases >400 mL following bronchodilator, consider asthma, or coexisting asthma and COPD. | III-2 | Strong |
| Further investigations may help a) confirm or exclude other conditions (either coexisting or with similar symptoms to COPD) and b) assess the severity of COPD. | III-2 | Strong |
| Referral to specialist respiratory services may be required | III-2 | Strong |
CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) is a preventable and treatable disease with some significant extrapulmonary effects that may contribute to the severity in individual patients. Its pulmonary component is characterised by airflow limitation which is not fully reversible. The airflow limitation is usually progressive and associated with an abnormal inflammatory response of the lung to noxious particles or gases (Global Initiative for Chronic Obstructive Lung Disease 2018). In clinical practice, diagnosis is usually based on:
- Symptoms of exertional breathlessness, cough and sputum
- A history of smoking, or exposure to other noxious agents
- FEV1/FVC<0.7 post-bronchodilator
Small-airway narrowing (with or without chronic bronchitis) and emphysema caused by smoking are the common conditions resulting in COPD. Chronic bronchitis is daily sputum production for at least three months of two or more consecutive years. Emphysema is a pathological diagnosis, and consists of alveolar dilatation and destruction. Breathlessness with exertion, chest tightness and wheeze are the results of airway narrowing and impaired gas exchange. The loss of lung elastic tissue in emphysema may result in airway wall collapse during expiration, leading to dynamic hyperinflation and consequent increased work of breathing.
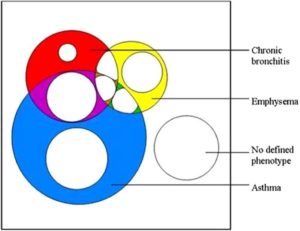
The irreversible component of airflow limitation is the end result of inflammation, fibrosis and remodelling of peripheral airways. Airflow limitation leads to non-homogeneous ventilation, while alveolar wall destruction and changes in pulmonary vessels reduce the surface area available for gas exchange. In advanced COPD there is a severe mismatching of ventilation and perfusion leading to hypoxaemia. Hypercapnia is a late manifestation and is caused by a reduction in ventilatory drive. Pulmonary hypertension and cor pulmonale are also late manifestations, and reflect pulmonary vasoconstriction due to hypoxia in poorly ventilated lung, vasoconstrictor peptides produced by inflammatory cells and vascular remodelling. The clinical features and pathophysiology of COPD can overlap with asthma, as most COPD patients have some reversibility of airflow limitation with bronchodilators. The follow up of a cohort of children aged 10 to 16 initially recruited in 1964 demonstrated that childhood participants who had wheezy bronchitis (n=53) and asthma (n=38) had an increased risk (OR 1.81 and 6.37 respectively) of COPD by mean age of 61, compared to cohort controls (n=239). Multivariate analysis details of adjustment for smoking were not provided (Tagiyeva 2016). A meta-analysis of six prospective cohort studies following children with or without wheezing into adulthood found an association between childhood atopic wheezing and prevalence of COPD in adulthood (RR 5.307, 95% CI 1.033 to 27.271, P=0.046) (Ma 2018). By contrast, some non-smokers with chronic asthma develop irreversible airway narrowing. The overlap between chronic bronchitis, emphysema and asthma and their relationship to airflow limitation and COPD are illustrated in Figure 1. This proportional Venn diagram presents data from the Wellington Respiratory Survey which recruited participants over the age of 50 and invited them to have detailed lung function testing and chest CT scans (Marsh 2008). It can be seen that almost all patients with both chronic bronchitis and emphysema meet the GOLD definition of COPD, as do most with both chronic bronchitis and asthma . Patients with chronic bronchiolitis, bronchiectasis and cystic fibrosis may also present with similar symptoms and partially reversible airflow limitation.
In recent years there has been a focus on the prevalence and implications of the co-existence of asthma and COPD. A systematic review and meta-analysis of 19 studies found that the prevalence of co-existing asthma in patients with COPD was 27% in population based studies and 28% in hospital based studies (Alshabanat 2015). Both this review and systematic reviews by Gibson (Gibson 2015) and Nielsen (Nielsen 2015) found an increased frequency of exacerbations in patients with features of both asthma and COPD compared to those with COPD alone.
Treatable Traits
Treatable Traits is a new treatment paradigm proposed for the management of people with airway diseases. The treatment approach has been suggested as a way to progress precision or personalised medicine in COPD and asthma (Agusti 2017, Agusti 2016, McDonald 2019b). Patients are first assessed through a detailed clinical history and identification of airway disease risk factors (e.g. smoking history, history of allergies, occupational exposures, family history, respiratory disease in early life); spirometry and measures of airway inflammatory biomarkers, including exhaled nitric oxide fraction (FeNO) and blood eosinophils. These assessments will indicate a high or low probability of the presence of an airway disease (Agusti 2016).
Following this confirmation, it is recommended that each individual undergoes a multidimensional assessment to identify treatable traits and an individualised treatment plan is implemented based on the presence of traits.
In order to be considered a trait, the following criteria should be met. Traits should be identifiable using a trait identification marker, clinically relevant and modifiable (McDonald 2019b).
Traits are grouped into three domains – pulmonary and extrapulmonary traits and behaviours/ risk-factors. While overall management according to treatable traits is a concept, the treatment of each individual trait is supported in most cases through RCT evidence. A proof of concept clinical trial in patients with COPD demonstrated that the treatable traits approach can lead to significant improvements in health-related quality of life (HRQoL) and inflammatory biomarkers (McDonald 2013) with similar results in an RCT in people with severe asthma (McDonald 2019a).
< Prev Next >