C1. Aetiology and natural history
Cigarette smoking is the most important cause of COPD (Fletcher 1977, Burrows 1977, Matheson 2018). There is a close relationship between the amount of tobacco smoked and the rate of decline in forced expiratory flow in one second (FEV1), although individuals vary greatly in susceptibility (Fletcher 1977). Around half of all smokers develop some airflow limitation, and 15 to 20% will develop clinically significant disability (Fletcher 1977). Even smokers who do not meet spirometric criteria for COPD may have respiratory symptoms and reduced physical activity. They may have other subtle abnormalities of lung function (Elbehairy 2016). Smokers are also at risk of developing lung cancer, and cardiovascular disease such as ischaemic heart disease and peripheral vascular disease.
In susceptible smokers cigarette smoking results in a steady decline in lung function, with a decrease in FEV1 of 25–100 mL/year (Fletcher 1977). While smoking cessation may lead to minimal improvements in lung function, more importantly it will slow the rate of decline in lung function and delay the onset of disablement. At all times smoking cessation is important to preserve remaining lung function (Fletcher 1977).
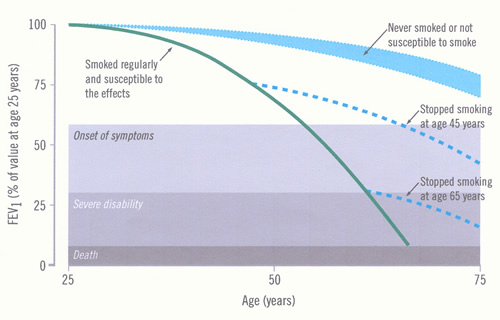
Impairment increases as the disease progresses, but may not be recognised because of the slow pace of the disease. The time course of development of COPD and disability and the influence of smoking cessation are illustrated in Figure 4.
The annual decline in FEV1 has been measured in 5,041 patients with moderate to very severe COPD followed for 4 years (Tashkin 2013). The decline in post-bronchodilator measurements was greater than pre-bronchodilator, which might represent progression of disease or tachyphylaxis [evidence level III-2].
Figure 4: Time-course of chronic obstructive pulmonary disease (COPD) (Fletcher 1977)
The figure (adapted from Fletcher C and Peto R. The natural history of chronic airflow obstruction. BMJ 1977;1:1645-1648 and reproduced with permission from the BMJ Publishing Group) shows the rate of loss of forced expiratory flow in one second (FEV1) for a hypothetical, susceptible smoker, and the potential effect of stopping smoking early or late in the course of COPD. Other susceptible smokers will have different rates of loss, thus reaching “disability” at different ages. The normal FEV1 ranges from below 80% to above 120%, so this will affect the starting point for the individual’s data (not shown). |
Exposure to second hand smoke (SHS) is also associated with increased risk of developing COPD. Chen et al in a meta-analysis 15 studies (6 cross-sectional studies, 6 case-control studies, and 3 cohort studies) with 25,592 participants found that SHS exposure was associated with an increased risk of COPD (OR 2.25; 95% CI 1.40 to 3.62, p < 0.01, I² = 98%, for heterogeneity based on a random-effects analysis model). The risk was higher in those with exposure of more than 5 years (OR 4.38; 95% CI 1.28 to 15.00, p < 0.01, I² = 89% for heterogeneity based on a random effects analysis model) (Chen 2023) [evidence level I].
Hookah (a type of water pipe) smoking is increasing, particularly in developing countries. In an Iranian study involving 245 adults aged ≥35 years who had at least 15 years of hookah smoking history and matching controls, the prevalence of COPD among hookah smokers was 10.2%; higher rates were found in older age, longer duration of hookah smoking; in men; history of ≥3 hookahs/day; history of cough for ≥2 years; history of sputum for ≥2 years; and a history of dyspnoea for ≥2 years (Bahtouee 2018).
In addition to cigarette smoking, there are a number of other recognised risk factors for COPD (Global Initiative for Chronic Obstructive Lung Disease 2024, Omland 2014) (see Box 2). COPD almost always arises from a gene environment interaction. The best characterised genetic predisposition is alpha1 antitrypsin deficiency, but multiple other genes each make a small contribution and further investigation is required. The risk of COPD is related to the total burden of inhaled particles and oxidative stress in the lung.
Analysing the lifetime job-histories of ∼100,000 individuals from a UK general population found that the following specific occupation categories are associated with an increased COPD risk: sculptor, painter, engraver, art restorer; gardener, groundsman, park keeper; food, drink and tobacco processor; plastics processor, moulder; agriculture, and fishing occupations not elsewhere classified; and warehouse stock handler, stacker. These associations were confirmed among never-smokers and never-asthmatics and were influenced by employment duration. Gathering job-history and focused preventive strategies in COPD high-risk jobs are warranted (De Matteis 2019).
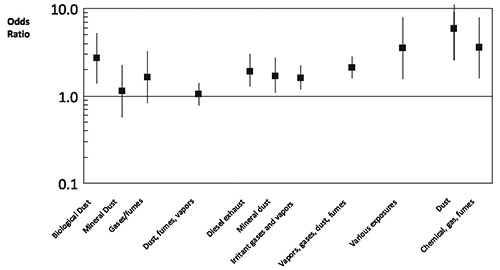
Occupational dust exposure might be responsible for 20 to 30% of COPD. This is consistent with the findings of a European study (Lytras 2018). This has long been recognised in coal miners (Santo Tomas 2011) but biological dust has also been identified as a risk factor, particularly in women (Matheson 2005). Non-smoking women involved in the spinning, weaving and knitting of cotton or silk have an increased risk of death from COPD (Cui 2011). Biological dust exposure is also associated with chronic sputum production, dyspnoea and work inactivity in male patients (Rodriguez 2008). Livestock farmers are also at increased risk of developing chronic bronchitis and COPD (Eduard 2009). Dairy farmers have increased wheeze and morning phlegm and increased rate of decline in FEV1 compared to controls. These effects appear to be associated more with exposure to animal feed than handling hay or straw (Thaon 2011). Lifetime cumulative exposure to pesticides is associated with risk of developing COPD (De Matteis 2022). Each year of exposure to diesel exhaust increases the risk of dying from COPD by 2.5% (Hart 2009). An analysis of a Swiss cohort of 4,267 patients without asthma found that COPD was associated with high occupational exposures to minerals, biological dusts, vapours/fumes, vapours, gases, dust or fumes (VGDF). The findings were clearer in non-smokers and those without chronic bronchitis (Mehta 2012) [evidence level III-2]. A meta-analysis of 6 cross-sectional studies found that occupational exposure to respirable quartz dust was associated with a pooled reduction in FEV1 of -4.62 (95% CI -7.18, -2.06) % predicted (Bruske 2014). A case control study conducted within a large managed care organisation found that self reported exposures to vapours, gas, dust and fumes on the longest held job were responsible for 31% of COPD (Blanc 2009). Joint exposure both to smoking and occupational factors markedly increased the risk of COPD [evidence level III-2]. Evidence of emphysema and gas trapping on CT scans was associated with self-reported occupational exposures to dust and fumes in both men and women who were former or current smokers (Marchetti 2014). A summary of the risks of COPD associated with biological or mineral dusts, gases, fumes / vapours, diesel exhaust, irritant gases / vapours, chemical gas / fumes and various other occupational exposures appears in Figure 5 (reproduced from Diaz-Guzman et al 2012 (Diaz-Guzman 2012) with permission).
Figure 5: Risk of occupational exposure for COPD from selected studies
|
Fortunately, the air quality in most Australian and New Zealand cities is relatively good and cooking with biomass fuels (coal, wood, dung, crop waste etc.) is uncommon. However, a panel study of 84 moderate to severe COPD patients found that indoor pollutant exposure, including PM2.5 and NO2 (oxides of nitrogen) was associated with increased respiratory symptoms and risk of COPD exacerbation (Hansel 2013) [evidence level III-2].
Prasad et al (2022) used modelling of exposure at an individual level and respiratory questionnaire and respiratory function testing data to examine the effect of a 6-week period of coal fire PM2.5 exposure from a 2014 Hazelwood open cut coal mine. A dose–response association between particle exposure and COPD in non-smokers and increased chronic cough in current smokers was observed [evidence level III-2].
Failure to achieve maximum lung function increases the risk of COPD in later life (Bui 2018, Lange 2015). Premature birth is associated with the development of COPD (Bui 2022). This association is compounded by smoking [evidence level III-B]. There is some evidence that women might be more susceptible to the effects of tobacco smoke (Aryal 2014) [evidence level III-2]. Beyond the age of 45-50 years, female smokers appear to experience an accelerated decline in FEV1 compared with male smokers (Gan 2006) [evidence level II]. On the other hand, a family-based case-control study involving high-resolution chest CT scans found that men demonstrated more low attenuation areas consistent with emphysema than did women (Camp 2009) [evidence level III-2] Nor is it known whether the increased risk among lower socioeconomic groups is due to greater exposure to pollution, poorer nutrition, more respiratory infection or other factors.
Novel risk factors for COPD have been reviewed by an assembly of the American Thoracic Society (Eisner 2010a). Exposure to second-hand (Environmental) Tobacco Smoke was consistently associated with various definitions of COPD; there was a temporal relationship, dose response gradient and biological plausibility. Meta-analysis of 12 studies found a pooled odds ratio of 1.56 (95% CI 1.40 – 1.74). There was sufficient evidence that exposure to smoke from burning biomass fuels was associated with development of COPD in women. Meta-analysis of 15 studies found a pooled odds ratio of 2.23 (95% CI 1.72 – 2.90), but there was significant heterogeneity between studies. [evidence level III-2]. Whilst the risk of biomass smoke in men has only been assessed in three studies, there also appears to be a similarly increased risk of COPD (OR 4.3, 95% CI 1.85-10) (Hu 2010). Pulmonary tuberculosis can lead to scarring and irreversible loss of lung function, however, there is currently insufficient evidence that this is clinically similar to COPD caused by cigarette smoking (Eisner 2010a). After extensive adjustment for potential confounders, a self-reported past history of TB had an adjusted odds of 3.78 (95% CI 2.87-4.98) of a diagnosis of COPD in a review of studies which were exclusively of low- and middle-income countries. This review comprised 12396 people aged 35 to 95, of cross-sectional data from 13 low- and middle-income countries and three continents. Overall prevalence of COPD was 8.8%, and those with a history of TB had an overall COPD prevalence of 25.9% (Kamenar 2021) [evidence level III-2]. The authors suggested that previously underestimated endobronchial spread and airway fibrosis as the mechanism.
In the Tasmanian Longitudinal Health Study, there were five different asthma /allergy trajectory patterns demonstrated in the prospective cohort of participants. This cohort included n=7380 initial participants at seven years of age, to n=2689 of the original participants at 53 years of age. Those with early onset-onset persistent asthma and allergies were most likely to develop COPD (OR 5.3, 95% CI 3.2-8.6.), followed by late-onset asthma and allergies (OR 3.8, 95% CI 2.4-4.6) (Bui 2021). This highlights the need for a personal approach including the management of treatable traits to potentially prevent progression to COPD. A past history of childhood asthma has been shown to be independently associated with a 3-fold (95% CI 2.25-4.00) increase in prevalence of adulthood COPD in a meta-analysis of 11 studies, which included 4294 people with and 44381 people without COPD (Ali 2022) [evidence level III]. Smoking status and other recognised risk COPD factors were adjusted for across this study.
Early life risk factors that could lead to lung problems in later life are discussed further by the European Lung Foundation: https://europeanlung.org/en/information-hub/keeping-lungs-healthy/early-life-risk-factors/
< Prev Next >